The Importance of pH in growing Cannabis
A grower aiming for quality cannabis needs to master every parameter to ensure the best growing conditions for his or her plants. One of these parameters is knowing the importance of PH in growing cannabis and pH of the growth substrate. To put it simply, the pH tells how acidic or basic the soil or your growth medium is. Others use the term ‘alkaline’ to describe high pH or basic soil, but they mean the same.

“If the plant looks a little bit pale, consider [taking] a sample to confirm [if] you have a problem with the pH.”
—– Pieter Klaassen, Horticulturist from CANNA
To understand pH easily, you can think that it affects your plants the same way that we are affected by acids and bases. Acids like vinegar and citrus juices taste sour and leave a stinging feeling on the skin. On the other hand, bases like soap and baking soda feel slippery and taste bitter. Exposure to strong acids and bases can cause injury and death in humans. The point we are emphasizing here is that plants need to maintain specific pH levels to stay alive and perform their best. In other words, the pH should not be too low or too high.
The pH scale ranges from values 1 to 14. The pH of pure water is at pH 7, which is called neutral pH. At this level, the liquid is neither acidic nor basic. Always remember that acids have pH lower than 7 whereas bases have pH higher than 7. In addition, the farther pH values are from pH 7, the stronger an acid or base is. In other words, the strongest acids have pH of 1 to 3 while the strongest bases have pH of 12 to 14. For example, sulfuric acid, which is a strong acid (pH 1 to 3), can cause severe skin burns, irritation, difficulty in breathing, blindness, and burn holes through tissues.
Pieter Klaassen from CANNA talks about the basics of pH in this video. We explain this further here to teach new and seasoned growers alike so you can master this parameter.
What is pH?

We can look at pH as the amount of acid or the amount of hydrogen ions (H+) per liter of water. In other words, increasing the hydrogens ions in the solution makes it acidic. The ‘pH’ in the pH scale actually stands for the power (or potential) of hydrogen. This means that the changes in hydrogen ion concentration occur in powers of 10 as we go along the scale. To illustrate
● pH 3 equals 1×10-3 hydrogen ions per liter of water, or 0.001 H+ ions / L
● pH 4 equals 1×10-4 hydrogen ions per liter of water, or 0.0001 H+ ions / L
● pH 6 equals 1×10-6 hydrogen ions per liter of water, or 0.000001 H+ ions / L
● pH 7 equals 1×10-6 hydrogen ions per liter of water, or 0.0000001 H+ ions / L
Note that as the pH value increases, we add more zeros before the concentration of H+ ions. A pH 3 solution (1×10-3) has 1 molecule of H+ per 100 liters of water while a pH 4 solution (1×10-4) only has 1 molecule of H+ per 1000 liters of water. This presents us with two important concepts to remember:
● The acidity of the solution INCREASES as the pH DECREASES. There are more hydrogen ions in a pH 1 solution versus a pH 3 solution
● A change of 1 in pH represents a 10 times change in hydrogen ions. For example, a pH 3 solution is 10x more acidic than a pH4 solution. On the other hand, a pH 3 solution is 10000 more acidic than a pH 7 solution.
Pieter gives a practical application of these principles in his talk. We start with two cups: one with pH 3 solution and the other at pH 7. What will be the resulting pH when we mix the contents of the two cups? It is wrong to simply get the average of the two values and result with a pH 5 solution. If we go back to what we have learned, we would see that we are actually combining a 0.001 H+ ions / L acid solution with a 0.0000001 H+ ions / L solution, giving us a 0.0010001 H+ ions / L acid solution. In this case, combining a pH 3 and pH 7 solution ends up with a solution of still about pH 3.
Ideally, pure water is neither an acid or a base because it has neutral pH. In reality, carbonates and bicarbonates are dissolved in tap water, which act as a kind of buffer. Buffers prevent drastic or rapid changes in the pH of the solution. Any drastic change in pH is life threatening to plants, so they need to have buffer systems to maintain the pH in a safe range as humans do.
Tap water pH

– Credit: @purecraftcannabis
Carbonates and bicarbonates in the tap water matter to growers because they can change the pH of the nutrient solution. The carbonates react with and decrease the acid in the nutrient solution, resulting in a more alkaline/basic solution (an increase in pH). If we look at the reaction below, the hydrogen concentration (therefore, the acidity) decreases because it is eventually converted to water and carbon dioxide (CO2). The principle behind this is similar to how the carbon dioxide in soda escapes when you first open the can. Eventually, the soda tastes less sour then more bitter because it becomes less acidic.
The trick here is to cover the nutrient mixture with a steel pot layer to prevent the escape of CO2 gas. When giving water, the CO2 trapped under the steel pot layer goes back in the nutrient mix, so the pH becomes stable.
Roots breathe!

– Credit: Canva Stock Photos
A plant itself can change the pH of its substrate because its roots breathe. The roots breathe in the sense that when they use energy and take in oxygen (O2), they release CO2 and water. The CO2 can become trapped in and can acidify the substrate. If the substrate becomes too wet, it will eventually acidify because the CO2 cannot escape.
Another process that changes the pH of the substrate is the electric load of the nutrients being uptaken by the plant. Positively loaded nutrients increase the pH of the substrate (becomes less acidic) while negatively loaded nutrients decrease it (becomes more acidic). Remember that plants live and perform best in the appropriate range. Therefore, there needs to be a balance among these processes.
“When we give water to the plant, actually we give it to the substrate and the roots are standing in that substrate.”
—– Pieter Klaassen, Horticulturist from CANNA
Growth phase matters
It would be a mistake to set the pH range of the substrate without first knowing the growing phase at which the plant is currently in. That’s why knowing the importance of pH in growing cannabis, the rate of respiration, and nutrient requirements at each phase are major factors that influence the grower’s target substrate pH. Nutrients are absorbed by the plant differently based on the pH. CANNA provides a useful guide on the absorption of the nutrients below.
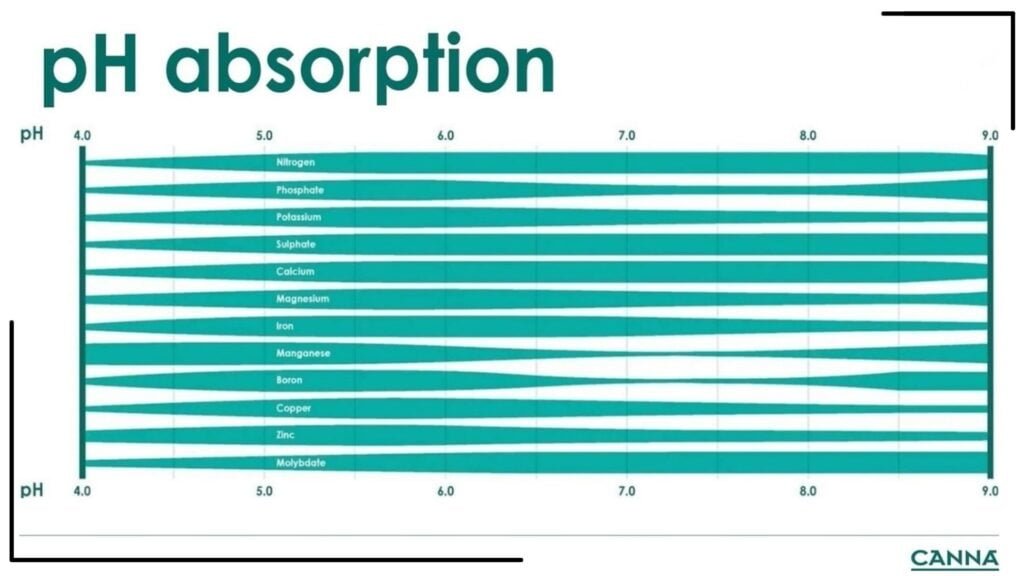
This guide lists the nutrients from top to bottom, while the pH scale is presented in increasing levels from left to right. It shows the relative absorption capacity of the plant for each nutrient per pH value. A thicker bar means that the plant has a higher absorption capacity for that nutrient at that specific pH value. We go over the pH changes and nutrient requirements per growing phase of cannabis below.
A. Vegetative Phase I and II
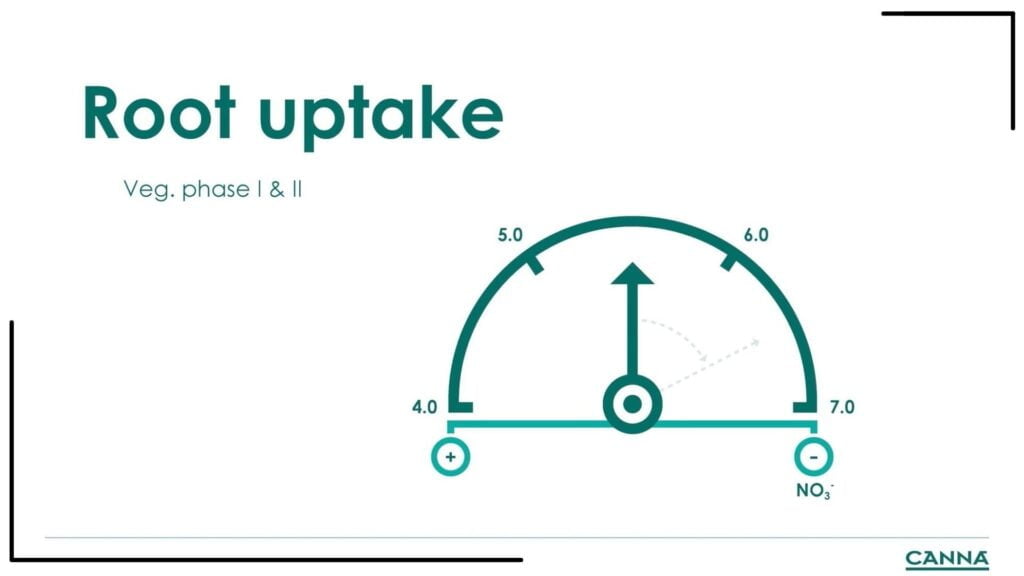
Cannabis gets a lot of nitrogen during the vegetative phases I&II, which increases the pH (less acidic). At pH 7.5, the plant can only do 50% of its maximum capacity for absorbing phosphate. Even when you give the right amount of phosphate, the plant will have phosphate deficiency because of poor absorption.
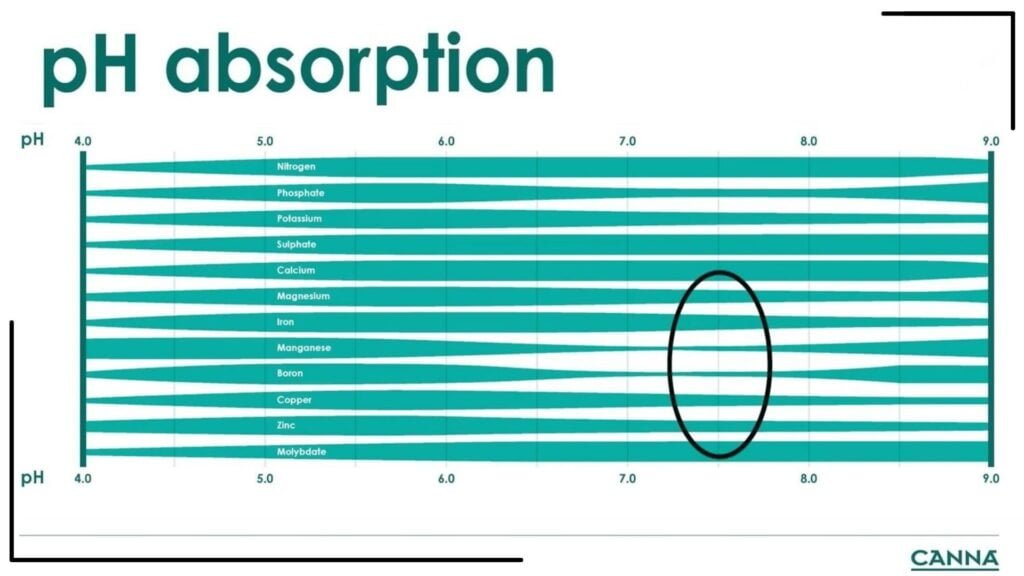
Other nutrients that are poorly absorbed in pH 7.5 are magnesium, iron, manganese, and boron. The deficiency of phosphates and trace minerals causes new leaves to become light green to yellow. Growers may see their plants in this condition and assume nitrogen deficiency when in fact, it is due to the deficiency of trace elements.
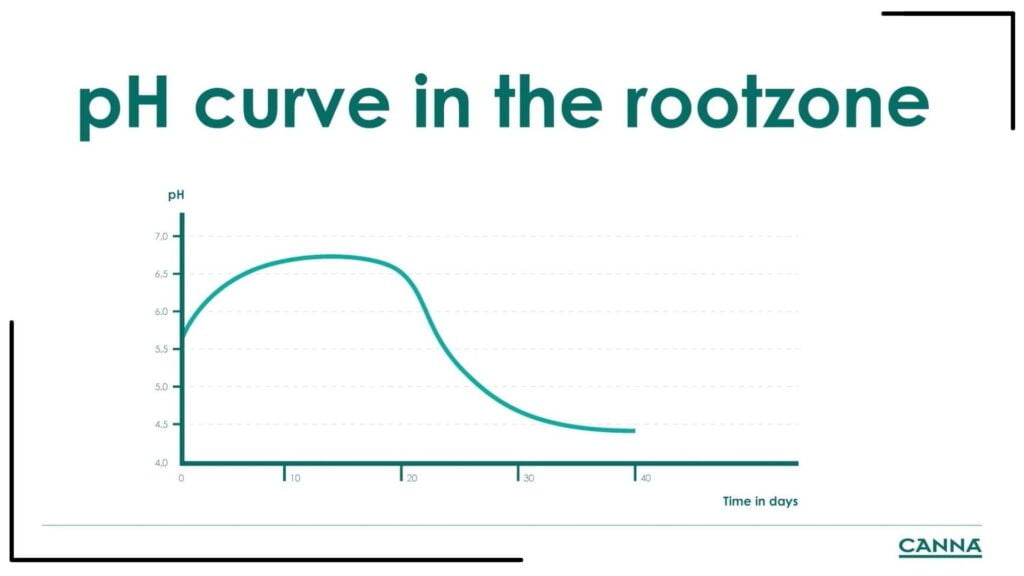
The graph shows the pH in the root zone of the plant as it goes through the vegetative phase. The pH goes up and plateaus to about pH 6.5 on days 10 to 20. After the vegetative phase, the pH flattens out and goes down eventually as the plant achieves balance with the positive loading of nitrogen and potassium when the grower gives fixative nutrients, like Canna Vega.
B. Generative phase I & II
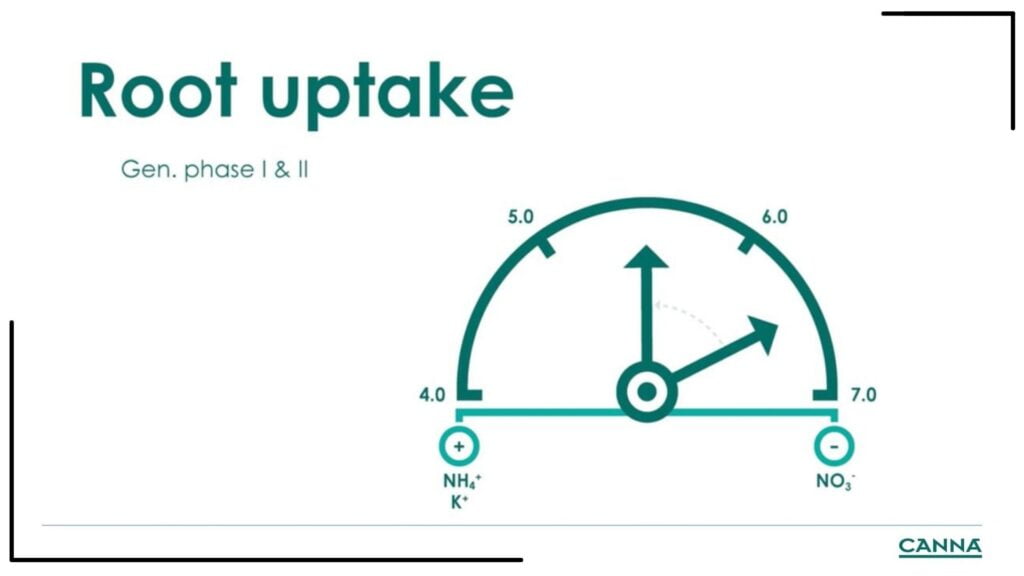
The plant needs more nutrients in the flowering phase, generative phase I or II. It can take 100% of the nutrients a grower gives at weeks three to five of the flowering phase. At this period, the pH stays between 5.2 to 6.2, which allows the maximum absorption of all nutrients by the plant.
C. Generative phase III
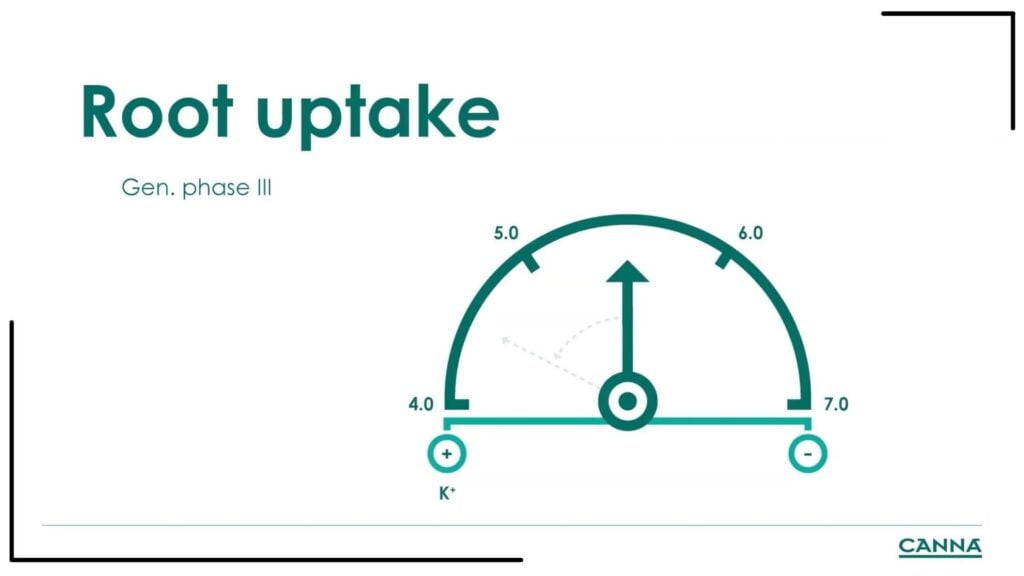
At week five, the pH in the root zone tends to go down to low levels (become more acidic) because the grower has started to give more of the flowering fertilizers, especially PK13,14 which contains a lot of potassium. The pH goes down to less than 5 during the generative phase III, so if we look at the pH absorption graph, it shows that none of the nutrients are available for the plant. The plant will be deficient in every nutrient because it cannot get them, except for manganese. The plant becomes dark green and very dull, and the grower should be aware that this is a sign of deficiency of multiple nutrients and manganese excess. Excess manganese may cause plant parts to break easily, and the leaves can show small brown spots next to the veins. The plant will still grow, but badly damaged leaves will fall off because of a lack of nutrients. Losing a lot of leaves because of low pH can impair a plant’s growth. That is why learning how to properly measure the pH is an essential component of any grower’s training about the importance of pH in growing cannabis.
“Because the plant is still willing to grow, the plant will sacrifice that leaf. The leaf is damaged so it takes everything out because it doesn’t get nutrients from the roots.”
—– Pieter Klaassen, Horticulturist from CANNA
How and when do you measure the pH?
In general, the pH reading should not be taken directly from the drain sample because the values can be misleading. The first drain from the substrate mostly contains the water that the grower gave. It is better to take readings from the second or third drains. We have an article on the parameter electrical conductivity explaining how to properly take drain samples.
If the grower gets a dark drain, it is likely that the pH reading may be a bit diluted. However, unlike other parameters, pH readings are less likely affected by dilution because it is on a logarithmic scale. Even if the sample is diluted 10 times, the pH value only goes up by one. Of course, it is still best to get a sample that closely represents the plant’s substrate. It is not always possible to get soil samples because doing so can damage the root zone, especially if the grower only has a limited number of plants. If that is the case, remember that three moments are very important in measuring the pH:
- The first week of flowering. The root zone pH can become high at this point, so if the plant looks a bit pale, consider taking a sample to check the pH.
- Week five. This time is when the grower decides to give PK13/14. He/she can give a full dose of PK when the pH is still high in the substrate. Otherwise, the dose can be decreased or none given at all if the pH is already low.
- Flowering week six. The grower checks for the effects of the PK13/14 on the pH of the substrate. If the pH has become too low, the grower can flush the excess nutrients using pH-controlled water. An alternative is to give high volume pH 7 water to compensate for the low pH in the substrate.
The Ideal pH

The ideal pH for the root zone is between pH 5.2 to 6.2. There are acceptable pH ranges that work for CANNA’s Terra, Coco/COGr, and Hydro/Aqua substrates. Take note that not all substrates can handle this low pH. For example, the acids in the potting mix Terra can react when mixed with lime. If the pH is lower than 5.8 (below the acceptable pH for Terra), the lime will break down and the potting mix will become 100% peat. Peat has a pH of four and cannabis cannot grow at this level. In fact, a lot of potting mixes are based on peats so growers use a hydroponic system so they could adjust the pH.
Coco and COGr are organic products that work in pH ranges of 5.5 to 6.2. As they are organic products, their structure can get damaged when the pH becomes too low. The grower should start giving these products in the vegetative phase when the pH is at least 5.5. They can increase the dose little by little until the pH reaches 6.2 in flowering at week five.
In hydroponic or recirculating systems, the grower can use the full pH 5.2 to 6.2 range. He or she can give nutrients beginning at pH 5.2 in the vegetative phase, up to pH 6.2 in the flowering phase, which is around weeks five to six.
Controlling the pH in aquaponics or peat mixtures works this way. However, it becomes different when using organic fertilizers.
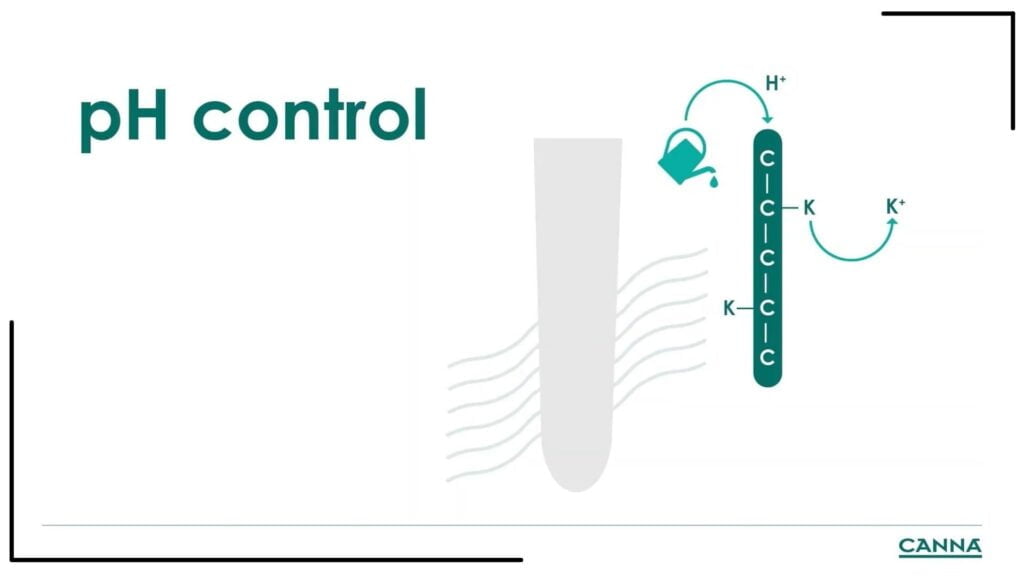
pH for organic fertilizers
The pH is not that important when using organic fertilizer because the plant only takes what it needs. As the roots breathe, the CO2 produced mixes with the water and creates acid. The acid then breaks down the organic structure of the fertilizer, which the plant then uses for nutrition.
“For organic fertilizer, it’s a totally different story. Actually, the pH is not important.”
—– Pieter Klaassen, Specialist from CANNA
Inorganic fertilizer has elements bound to it, like potassium, which is broken down by the acid. The plant will absorb the potassium but the positive electrons would not build up. In other words, the difference when using organic fertilizers is that the root decides which elements it wants to have. You still have to watch out though if you are controlling the pH of the water, because acids in water can break down the organic fertilizer. The plant can still grow on broken-down organic fertilizer but, of course, it would not get the best nutrition. Too much acid in the water can also break down excess nutrient elements from the organic fertilizer, potentially causing an overdose.
pH for hard water
In general, never control the pH of the water when using organic fertilizers like BIOCANNA. There is one exception, and that is if the grower is using very hard water. Hard water contains carbonates and bicarbonates that can neutralize the acid from the roots. Without the acid, though, the root becomes unable to break down the organic fertilizer and cannot get the potassium it needs. To solve this problem, check that before you start making the nutrient solution, add some acid to the water to reach a pH level of six. Leave the solution for at least 12 hours before mixing with the organic fertilizer. Although the pH would go up, you would not need to control the pH anymore. To reiterate, only acidify the nutrient solution when you are using organic fertilizer with hard water.
Your plant’s pH requirements will vary depending on the growth phase, the type of substrate you use, and the type of water you give your plants. As a grower aiming for quality, you also need to master every parameter, including knowing the importance pH in growing cannabis, to ensure the best growing conditions for your plants. Never too much and never too little; find the balance in everything.
Watch this session at The Grower’s Source Expo here.
Featured Image Credits: Canva Stock Photos

Want to keep the discussion going?
Log onto The Grower’s Source App to ask a question of our group of experts, or to read up on the latest comments on this topic.
You can also engage your fellow master and hobby growers about many other cannabis topics on your Grower’s Source App.
CONNECT
Connect and maintain relationships between you and other Canadian cannabis industry growers.
COLLABORATE
Overcome challenges together with your fellow growers, learn, develop collective knowledge and a global competitive edge.
GROW
Grow better, aim for sustainability, quality, and cost-effectiveness.








